- Overview
- Course outline
- Entry
- Career path
Course Overview
This programme is designed to equip participants with the essential knowledge and skills required to excel in quality assurance within the manufacturing industry. Consisting of two specialised modules, this programme delves into critical aspects of manufacturing operations and quality assurance standards.
This programme is specifically designed for learners seeking a qualification that will enable them to either progress within their current employment or take up employment in a manufacturing operations industry.
Designed and delivered by highly experienced academics and industry professionals, our lecturers know the skills and knowledge that companies are looking for. Our unique part-time, online delivery allows learners to log on live or catch up by watching the recorded sessions which can also be used for revision. Extensive coaching and mentoring workshops will be provided with a specific focus on individual assessment and improvement.
Accreditation: QQI Level 5 Certificate in Quality Assurance Essentials for Biopharmaceutical Manufacturing (30 FET)
In Partnership with: Mayo, Sligo and Leitrim Education and Training Board
Next Intake: May 2025. Now accepting application.


Continue to the Course Outline section for module information.

Course Outline
The course duration is 5 months. This part-time programme is delivered in a blended format. Lectures are delivered online in the evenings, with 3 in-person lectures taking place on Saturdays throughout the programme.
Module 1: Cleanroom Operations and Plant Utilities/Facilities
- Facilities & Utilities Management Overview:
– Efficient facility and utility management
– Maintaining a controlled environment. - Building Management:
– Management of cleanroom buildings
– Air pressure differentials, HVAC systems, and
cleanroom construction materials - Total Productive Maintenance
– The benefits of TPM
– Minimising unplanned downtime and operating at peak performance - Introduction to Environmental Health & Safety Management:
– Hazard identification, risk assessment, and mitigation strategies
– The importance of safety measures - Introduction to Regulations:
– The regulatory framework
– Regulatory agencies, guidelines, and standards relevant to different industries
– Legal and compliance
Module 2: Essentials of Quality Assurance
- Quality Management Systems (QMS):
– The history and evolution of QMS
– Regulations
– Key concepts
– GMP and compliance
- Documentation:
– GDP – practical current guidelines
– Technical writing
– Data integrity
– Case studies - Health & Safety
– Regulations
– Hazards and risks
– Risk assessment and management
– Personal protective equipment (PPE)
Entry Requirements
Proficiency in English Language Required (B2+ level or equivalent on the Common European Framework or Reference for Languages (CEFR).
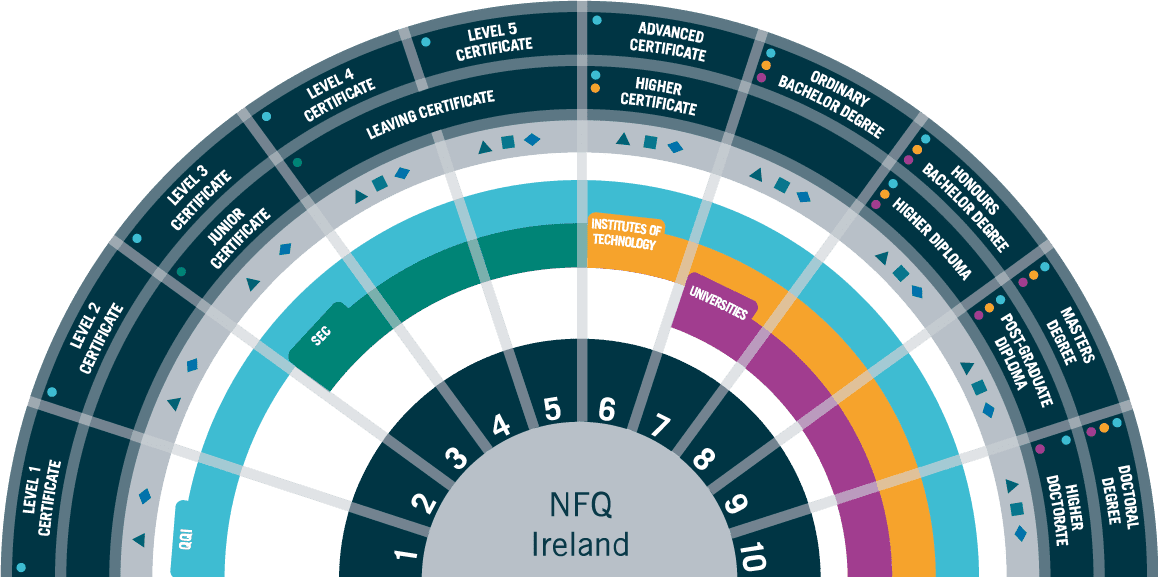
Career Path & Progression
Progression
Learners may complete additional Innopharma Education QQI Micro-Credential programmes and work towards completing a Level 6 Certificate in Operational Excellence for Digitisation (Minor Award with 60 ECTS) or Level 6 Certificate in Information Technology for Digitisation (Minor Award with 60 ECTS) or Level 6 Higher Certificate in Science in Process Digitisation (120 ECTS).

Why up-skill for the STEM process manufacturing sectors in Ireland
- €3 billion in new capital investment
- €39 billion in exports
- Over 55,000 employed
- 9 of top 10 world’s biopharma companies in Ireland
- 12 of the top-selling drugs made in Ireland
- Over 8,000 new jobs predicted by 2020

Testimonials
The lecturers are all experts in the food industry, they bring us practical experience, and equip us with many skills to sharpen our edge in the job market. Â I would recommend Innopharma to anyone who is interested in advancing their career in the food industry.
Lynn Chen McGrath, MSc Food Business Management & Technology Graduate
Our Blog
Blog
April 17, 2025
The EU AI Act: What It Means for Medtech (and Why You Should Care)
The EU AI Act Countdown Begins The countdown is on for the application of the EU’s Artificial Intelligence (AI)...
Blog
January 30, 2025
AI Shakeout: How DeepSeek and Alibaba Are Rewriting the AI Landscape
Author: Robert Farrell, Program Director – Digital Transformation & AI, Innopharma Education The AI landscape is experie...
Blog
December 17, 2024
Digital Transformation 101: What is Industry 5.0?
Industry 5.0 is the term coined for the next evolution in digital transformation, emphasising the importance of human-centred appr...






















