- Overview
- Course outline
- Entry
- Career path
- How to apply
Course Overview
This Honours Degree is suitable for those who wish to develop their career in the areas of pharmaceutical operations, compliance, business improvement, technology transfer, validation and clinical trials. With extensive input and participation from the pharmaceutical and clinical trial sectors, this programme is ideal for those involved in manufacturing, compliance, business improvement, quality assurance, validation, data analytics, clinical trial coordination, validation, data analytics and clinical coordination.
The pharmaceutical industry has traditionally required individuals with a strong science or engineering background to support its significant manufacturing activities in Ireland. Today, there is a new skill set required along with those with scientific knowledge. This skill set requires professional individuals with a strong knowledge of manufacturing, compliance, regulation as well as business improvement. These skills need to be supported with an ability to assimilate information, make effective decisions and communicate clearly using the latest technologies and systems.
Modules Include:
- Pharmaceutical Manufacturing
- Management & Organisational Behaviour
- Operations Management
- Regulatory Affairs & Validation
- Operational Excellence – Lean Sigma
- Control Systems and Real-Time Analytics
- Clinical Research Co-Ordination
- Pharmaceutical Quality Management Systems
- Pharmaceutical Manufacturing & Distribution
- Strategic Management
- Clinical Research
- Project Planning & Finance
- Professional & Technical Communications
This BA (Hons) in Pharmaceutical Business Operations developed by Innopharma Education in conjunction with Griffith College is suitable for those with a higher certificate in science, engineering or a related discipline who are looking to start a new career or develop an existing one. Designed and delivered by highly experienced academics and industry professionals, you will learn the skills and knowledge that companies are looking for.
This course is delivered through blended learning, 2 evenings per week online and up to 2 Saturdays per month in person.
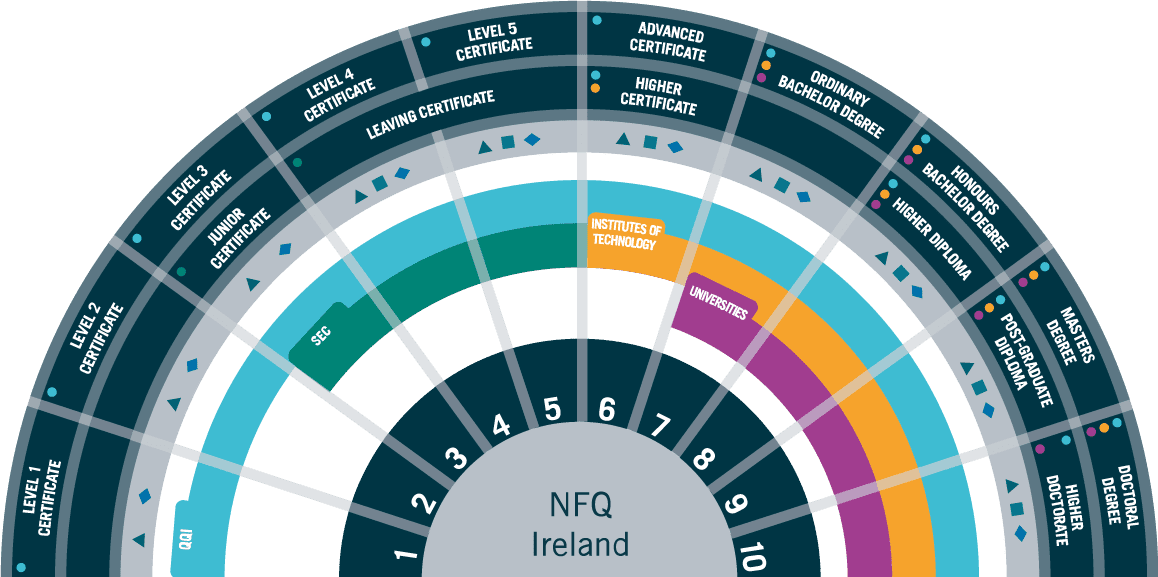
Accreditation: QQI Level 8 BA Honours Degree in Pharmaceutical Business Operations.
Collaborating College: Griffith College Dublin
Next Intake: January 2026. Now accepting applications.


Course Outline
This course is delivered over 2 years. Students have the option to enter at year 1 or year 2 depending on their prior qualifications. (ECTS: 120 Credits)
Year 1
Module 1: Pharmaceutical Manufacturing
Pharmaceutical dosage forms; drug product formulation and their chemical function; pharmaceutical manufacturing processes; quality control release testing requirements; stability testing; process validation; change control
Module 2: Management & Organisational Behaviour
Organisational structure and design; organisation culture and change; management & leadership; organisational control and power; workgroups and teams in organisations
Module 3: Operations Management
Operations management; sales & operations planning; planning control and improvement; performance measurement; operational governance
Module 4: Regulatory Affairs & Validation
Regulatory requirements for pharmaceutical manufacturing; distribution and clinical trials; elements of typical cGMP quality systems; clinical trials; pharmaceutical manufacturing; pharmaceutical distribution; marketing authorisation application; current trends in cGMP.
Module 5: Operational Excellence – Lean Sigma
Lean thinking; six sigma; project planning; process mapping; root cause analysis; creative solutions; corrective action preventative action; statistics; data visualisation; presentation skills; effective decision making
Module 6: Control Systems and Real-Time Analytics
Control and data management systems; system development lifecycle; process analytical technologies; real-time control system; critical quality attributes
Module 7: Clinical Research Co-Ordination
Research & development process; clinical studies; statistical concepts in a clinical study; drug safety system; regulations & directives applicable to clinical trials; GCP inspection; clinical study reports & clinical overview
Module 8: Career Progression
Fundamentals of developing a successful CV; cover letters; presentation and communications skills; networking; interviewing skills; personal coaching & mentorship
Year 2
Module 1: Pharmaceutical Quality Management Systems
Pharmaceutical quality systems; validation; change control; investigations; audit performance; annual product reviews (APR); quality metrics; quality risk management.
Module 2: Pharmaceutical Manufacturing & Distribution
Biopharmaceutical manufacturing overview; pharmaceutical packaging; good distribution practice; product release; falsified medicines directive.
Module 3: Strategic Management
Introduction to strategic management; scanning the environment; strategy formulation; strategy implementation & change management; evaluation and control.
Module 4: Clinical Research
Drug development lifecycle; phases of clinical research; primary regulations & directives; GCP auditing/pharmacovigilance; clinical pharmacology.
Module 5: Project Planning & Finance
Project management; project initiation & conceptualisation; project scheduling and time management; project budgeting/costing; project closure & communication; project leadership & communication; risk management.
Module 6: Professional & Technical Communications
Career preparation skills; CV development; Interview skills; technical communications.

Entry Requirements
This course is suited for those with a minimum qualification of Certificate at QQI Level 7 or higher in Science, Engineering, Quality or a related discipline who wish to develop their knowledge and skills on the application of science in the Pharmaceutical industry.
Applicants with a Level 7 Degree in a relevant discipline and a minimum of 3 years of relevant work experience are also eligible to apply under the college’s Recognition of Prior Learning policy.
Career Options
Potential career opportunities following the successful completion of this course are highlighted below:
- Manufacturing Supervisor / Manager
- Supply Chain Planner
- Clinical Trials Coordinator
- Business Improvement Specialist
- Lean and Six Sigma
- Compliance Specialist
- Process Improvement Specialist
- Project Co-ordinator
- Pharmaceutical Operations
Academic Progression
The options for further study upon successful completion of this course could include progression to the following postgraduate course:
- MSc in Digital Transformation
- PG Dip in Pharmaceutical Business & Technology
- MSc in Pharmaceutical Business & Technology
- PG Dip in Medical Device Technology & Business
- MSc in Medical Device Technology & Business
- PG Dip in Digital Transformation (Life Science)
Apply Now
Our admissions team are on hand to assist you with your application and answer any questions you may have on the course.
Students who wish to apply to this course should follow the following application process.
Step 1: Enquire through the form at the top right of this page
Step 2: A member of our team will be in contact through phone or email
Step 3: If you are deemed to be eligible for the course, you will be sent an application form by email. You MUST fill in this application form and attach all necessary *documents (CV, ID, Transcripts, etc.)
Step 4: On submission of the completed application form and all documentation, your application will be sent to the associated department for final approval. You will receive an e-mail confirming your place on the programme. (Note: This process can take up to one week particularly during busy admission periods.)
*We accept scanned documents in a pdf format. Pictures of documents are not accepted.
**For those who are applying for Springboard funding, you will be directed to fill out an additional application form on the Springboard website, this is to confirm your eligibility to receive funding
Please note at any time if you have any questions please do not hesitate to contact us by email on admissions@innopharmalabs.com or call us (01) 485 334
Why up-skill for the pharmaceutical industry in Ireland?
- €3 billion in new capital investment
- €39 billion in exports
- Over 55,000 employed
- 9 of top 10 world’s biopharma companies in Ireland
- 12 of the top-selling drugs made in Ireland
- Over 8,000 new jobs predicted

Testimonials
There is content in every module that I use as part of my job every day
BA in Pharmaceutical Business Operations Graduate, Sabrina Mitchell, People Team Lead in GE Healthcare
Our Blog
Blog
April 3, 2025
AI in Biopharma: Transforming Manufacturing & Innovation
How AI, Machine Learning, and Automation are Transforming Biopharmaceutical Manufacturing Here at Innopharma, we are proud to show...
Blog
December 11, 2024
Looking for a Career in the Biopharmaceutical Industry? Everything You Need to Know
The biopharmaceutical industry is a rapidly growing sector that combines cutting-edge biotechnology with pharmaceutical science to...
Blog
October 18, 2024
What is Pharmaceutical Manufacturing & Why is it Important?
Pharmaceutical manufacturing is the name given to the production of medicinal products on an industrial scale. Pharmaceutical/Biot...






















