- Overview
- Course outline
- Entry
- Career path
- How to apply
Course Overview
This Higher Diploma is specifically designed to enable those, with a qualification in science or engineering, to successfully transition into technical or professional roles within the Biopharmaceutical and Medical Device sectors and to upskill those working in the sector to fill existing and emerging skills needs of the industry.
This programme combines a blend of Pharmaceutical and Medical Device Manufacturing modules with a range of business and industry-directed modules. Designed and delivered by highly experienced academics and industry professionals, you will gain the advanced skills and knowledge that companies are looking for. Our professional development team will help you all the way. We’ll help you to tailor your CV towards an area that you are interested in getting a job in and use our industry contacts to help you get your foot in the door.
Modules covered:
- Aseptic processing and utilities
- Regulatory affairs & GMP
- Medical Device operations
- Bioparhamceutical process
- Pharmaceutical Process Chemistry
- PAT & Process validation statistics
- Finished dose formulation and manufacturing
- QRM principles in pharma and medical device
- Lean operations and deviation investigation methods
- Communication skills and work preparation
With our unique part-time online delivery, learners receive minimum disruption to their schedule. Learners can log on live or catch up through recorded sessions when suited.
Accreditation: QQI Level 8 Higher Diploma in Biopharmaceutical and Medical Device Manufacturing.
Collaborating University: Technical University Dublin – Tallaght Campus
Next Intake: Summer 2025. Now accepting applications.


Course Outline
Lectures are delivered 2 evenings per week Online and 2 Saturdays per month on campus. Labs are held in one of our pilot facilities with times and dates subject to scheduling. (ECTS: 60 Credits)
Course Modules Include:
Module 1: Aseptic Processing and Utilities
Provides the student with the skills and knowledge required for an Aseptic Manufacturing Environment.
Module 2: Regulatory Affairs and GMP
In-depth study of the Regulatory and GMP requirements of the pharmaceutical and medical device industries.
Module 3: Medical Device Operations
Medical Device, Design and Development, Regulations, Technologies, Packaging and Sterilization.
Module 4: Biopharmaceutical Processes
Equipment and processes of biopharmaceutical manufacture, including upstream/downstream processing.
Module 5: Pharmaceutical Process Chemistry
In-depth study of API production, focusing on scale-up, process optimization and unit operation.
Module 6: PAT and Process Validation Statistics
Requirements for validation of GMP equipment and facilities with hands-on experience of SPC and PAT.
Module 7: QRM Principles in Pharma and Medical Device
Evaluate and implement QRM strategies, ensuring compliance within these regulations.
Module 8: Lean Operations and Deviation Investigation Methods
Includes Lean and Six Sigma, CAPA, effective report writing and preparation for regulatory inspections.
Module 9: Finished Dose Formulation and Manufacturing
In-depth understanding and hands-on experience of drug formulation, manufacturing and packaging.
Module 10: Communication Skills and Work Preparation
Communication, leadership and career development skills for success in the life science sector.

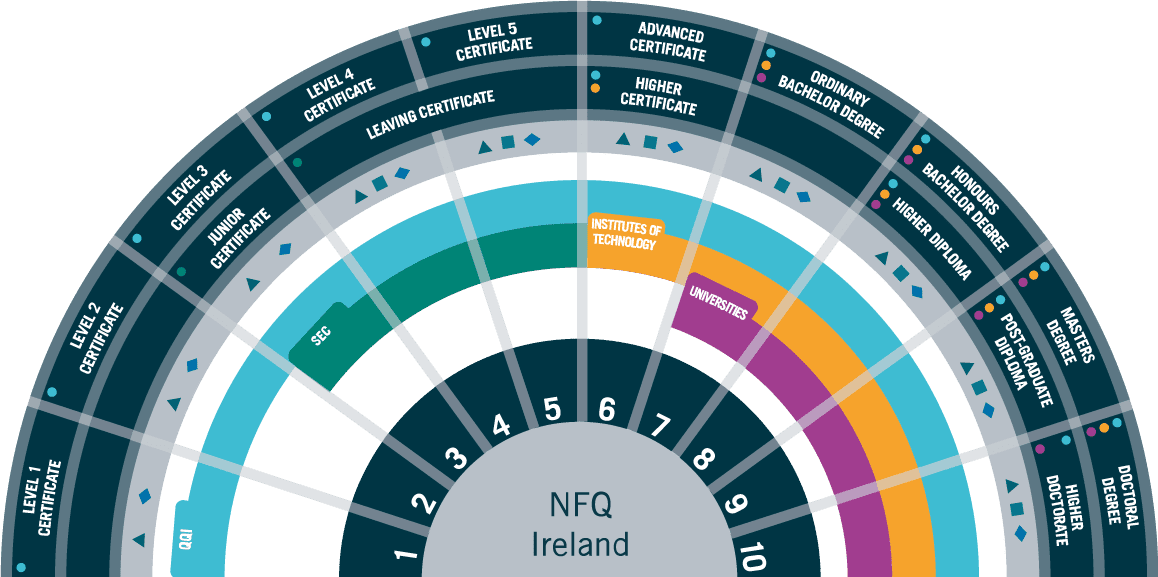
Entry Requirements
This course is suitable for those with higher-level qualifications at NFQ Level 7 (180 ECTS Credits, typically 3 years of study) in science, engineering or relevant discipline.
Career Options
With the continued growth of the Pharmaceutical and Medical Device industry in Ireland, this course is suitable for those who wish to upskill to gain either:
A technical position within the Pharma/Medical Device Industries in roles such as QC, QA, Project Management, Engineering, Managerial, etc.
or
Supplement their existing core skills to pursue a similar role within the Pharma/Medical Device Industries in areas such as Finance, HR, IT etc.
Potential Roles Include:
- Project Engineer
- Product and Process Developer
- Production Technician/ Operator
- Maintenance Support
- Quality Assurance Analyst
- Process Technicians
- Support Services
- Medical vigilance / surveillance and risk management
- Process business improvement specialists / managers
- Quality assurance specialist / manager
Apply Now
Our admissions team are on hand to assist you with your application and answer any questions you may have on the course.
Students who wish to apply to this course should follow the following application process.
Step 1: Enquire through the form at the top right of this page
Step 2: A member of our team will be in contact through phone or email
Step 3: If you are deemed to be eligible for the course, you will be sent an application form by email. You MUST fill in this application form and attach all necessary *documents (CV, ID, Transcripts, etc.)
Step 4: On submission of the completed application form and all documentation, your application will be sent to the associated department for final approval. You will receive an e-mail confirming your place on the programme. (Note: This process can take up to one week particularly during busy admission periods.)
*We accept scanned documents in a pdf format. Pictures of documents are not accepted.
**For those who are applying for Springboard or HCI funding, you will be directed to fill out an additional application form on the Springboard website, this is to confirm your eligibility to receive funding
Please note at any time if you have any questions please do not hesitate to contact us by email on admissions@innopharmalabs.com or call us (01) 485 334
Why up-skill for the pharmaceutical industry in Ireland?
- €3 billion in new capital investment
- €39 billion in exports
- Over 55,000 employed
- 9 of top 10 world’s biopharma companies in Ireland
- 12 of the top-selling drugs made in Ireland
- Over 8,000 new jobs predicted

Testimonials
As I already had a manufacturing background, I felt like it was necessary to have a course which could help me upskill my knowledge related to the pharma industry. Not only would I recommend, but I have already recommended Innopharma to a lot of people.
Zsolt Egerszegi, Manufacturing at Mylan
Our Blog
Blog
April 3, 2025
AI in Biopharma: Transforming Manufacturing & Innovation
How AI, Machine Learning, and Automation are Transforming Biopharmaceutical Manufacturing Here at Innopharma, we are proud to show...
Blog
December 11, 2024
Looking for a Career in the Biopharmaceutical Industry? Everything You Need to Know
The biopharmaceutical industry is a rapidly growing sector that combines cutting-edge biotechnology with pharmaceutical science to...
Blog
October 18, 2024
What is Pharmaceutical Manufacturing & Why is it Important?
Pharmaceutical manufacturing is the name given to the production of medicinal products on an industrial scale. Pharmaceutical/Biot...






















