‘Living Drugs’ & ‘Magic Bullets’: Cell & Gene Therapies Coming to Ireland

Dr Gillian McMahon looks at the developments in cell and gene therapies and what opportunities it brings for Ireland and Irish patients.
The human race has come a long way in drug development since people chewed the bark of the willow tree for pain relief. In the past century, we have seen medicine change from elixirs of antibiotics all the way to the most recent ‘magic bullet’ cancer treatments. The FDA approved the first immunotherapy agent in 1986 (interferon) and the EU approved the first gene therapy in 2012 (Glybera). Since then, immunotherapies have become even more targeted and customised.
Ireland has 19 of the top 20 global pharma and biopharma companies based here and the sector is worth over €100 billion to the Irish economy each year. As a country, Ireland is currently ranked 12th in global scientific rankings, with a strong track record in areas including immunology and pharmacology. Cell & Gene Therapy (CGT) and advanced therapeutics are at the forefront of emerging technologies in healthcare and together represent one of the principal Strategic Themes of the BioPharmChem Ireland Strategy Document (2023-2027). Here, the focus is on growing ‘Ireland’s potential in next-generation biologics including the development of a thriving indigenous and start-up ecosystem’.
CAR-T for lymphoma and leukemia
One very exciting breakthrough in CGT is a new immunotherapy called chimeric antigen receptor – T cell (CAR-T), which is approved for patients with certain types of cancers including lymphoma and leukemia. It is a very complex treatment with a number of steps. Firstly, the patient’s own T-cells are harvested in a process called leukapheresis. These cells are then genetically engineered in the lab to express proteins called chimeric antigen receptors (CARs) which will target the surface of the specific cancer cells in the patient. The customised cells are then multiplied before being infused back into the patient’s bloodstream where they proceed to attack the cancer.
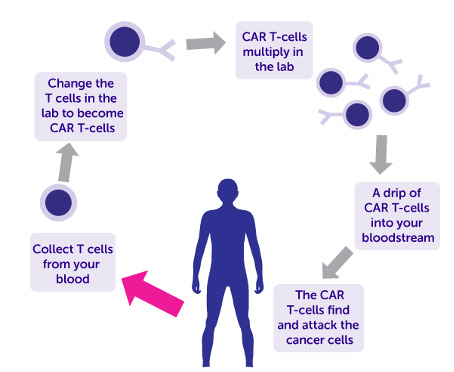
Figure 1:Diagram of how chimeric antigen receptor – T cell (CAR-T) works
(from Cancer Research UK)
The modified cells remain in the body for a long time, which is why CAR-T therapy is sometimes called a ‘living drug’. This treatment has now reached our shores and only last year, Ireland’s first adult received CAR-T therapy in Trinity St James’s Cancer Institute. It has also been administered to children with acute lymphoblastic leukaemia (ALL). A 2021 study from the US looked at the outcomes of children with relapsed ALL who were given CAR-T. Approximately 60% of the children were still alive five years later without their cancer returning. Due to results like this, this revolutionary new approach is now available to Irish children at Crumlin and Temple Street hospitals.
So, where is CAR-T heading next?
One of the newer applications for CAR-T is the treatment of solid tumours, which have traditionally been more challenging for immunotherapy. The range of diseases where CAR-T might be useful, not just in cancer, is also being studied. Recently, CAR-T cells were found to be effective at treating mice with an autoimmune disease like multiple sclerosis. Another very interesting strategy for CAR-T therapy is the use of T-cells from healthy donors instead of patients – this ‘off-the-shelf’ CAR-T therapy would be immediately available to patients rather than having to be engineered for each individual patient from their own cells, a process that currently takes weeks. It would also be less costly. The next generation of CAR-T will incorporate gene editing tools such as CRISPR in order to enhance the efficacy of the treatment and reduce toxicity in patients. This is especially important since a known serious side effect in some cases is cytokine release syndrome (CRS), which can be very severe, and anything that can reduce the chances of this happening would be welcome. For the six FDA-approved CAR-T therapies, treatment often leads to long-term survival, and in some cases, has even cured patients. Expectations are high and the availability of CAR-T is a very positive development for Irish patients and offers hope where previously there was very little.
What is Mitochondrial donation treatment?
Another exciting development in CGT is procedure called mitochondrial donation treatment (MDT). Earlier this month, it was announced that the first UK baby with DNA from three people was born using MDT. Although not the first baby in the world born using MDT – the first was born in Mexico in 2016 – the UK is the first country in the world to regulate the technology. Mitochondria are present in almost all of the cells in our body and are the ‘batteries’ which supply energy for the cells to do their work. They also carry a small amount of DNA of their own. Any abnormalities in this mitochondrial DNA would be passed from mother to child and can cause severe, life-limiting disorders such as Leigh syndrome or Alper’s disease. For women who have these rare, hereditary mitochondrial diseases, MDT means that they can now become pregnant without passing the faulty genes onto their children. There are two versions of the technique approved by the Human Fertilisation and Embryo Authority (HFEA) in the UK but both involve combining the healthy nucleus from the mother’s egg with healthy mitochondria from a donor. In the MDT approach shown in Figure 2 below, the modified egg then goes on to be fertilised in vitro by the father’s sperm.
‘Three parent babies’
Although the headline ‘three parent babies’ has been widely used in the media, it is important to understand that the donor is not a genetic parent in MDT. This is because most of the baby’s DNA comes from their mother and father, with less than 0.2% from the donor. Hence, the donor would not have any legal rights or responsibilities over the child and will remain anonymous.
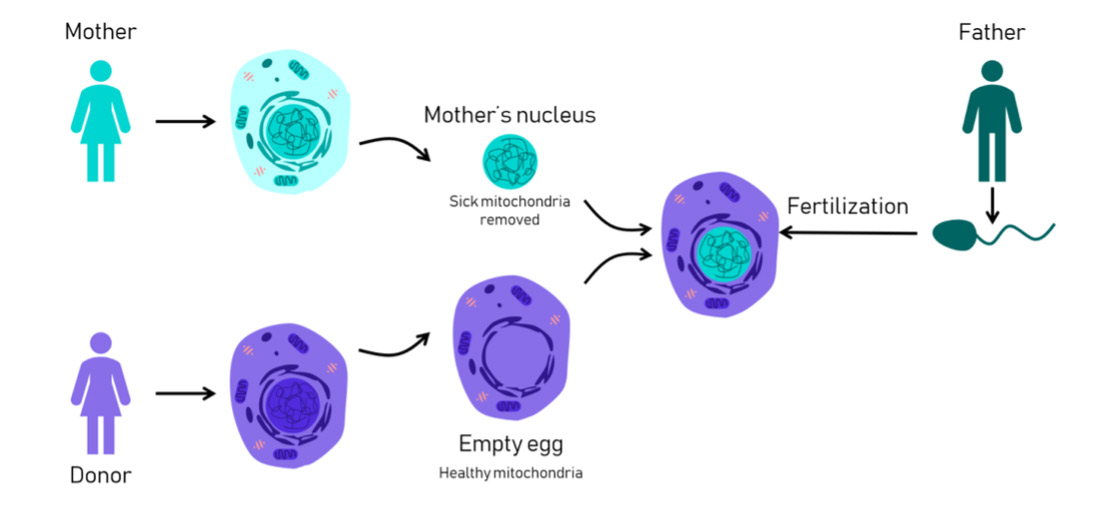
Figure 2:Diagram of how Mitochondrial Donation Treatment (MDT) works
(from Science in the News, Harvard University, Aug 2018, figure by Rebecca Clements)
While MDT offers families with severe mitochondrial illness the possibility of a healthy child, it is still in the very early stages of rollout with a number of challenges still to overcome. It is not yet clear whether the babies born using MDT will remain completely free of mitochondrial disease as they grow into adults. The parents of the first MDT baby born in Mexico in 2016 declined long-term monitoring of their child and it will be a few years before the long-term health of the UK children born using MDT will be available. Some countries remain cautious and are still debating the ethical, legal, cost and safety implications of the technique. Some of these concerns may be addressed by Monash university in Australia which is coordinating the second ever clinical trial on MDT. The trial will allow affected women to improve their chances of having healthy children but its success will depend on the willingness of Australian women to donate their eggs for the programme. It is predicted that, by stopping the inheritance of mitochondrial disease, MDT could save $500 million AUS in future healthcare costs over ten years.
So, where is MDT heading next?
Hopefully it will be available in Ireland soon so that families here can opt to have children free of the affected genes. Researchers are very optimistic about the future opportunities that MDT approaches might offer for parents with other hereditatry conditions (not just in mitochondrial DNA) and for women with fertility issues. It is likely that the procedure will rapidly find new medical applications in these and related fields.
So what is Ireland doing in CGT?
We have established a new National Advanced Manufacturing Centre in Limerick which has already started to work with the advanced therapeutics sector and NIBRT began construction last year on their new advanced facilities for CGT, mRNA-based therapies and other novel vaccines. Takeda have invested €60 million into Ireland’s first stem cell therapy state-of-the-art facility and have committed a further €36 million to support expansion. Ireland’s advanced manufacturing sector is worth about €140 billion in exports and employs over a quarter of a million people. In conjunction with our strong research environment, skilled workforce and supportive government policies, Ireland could become a centre of excellence for CGT.
In summary, CGT treatments offer huge potential. Some are even curative, such as Luxterna for inherited retinal blindness and Zolgensma for infants with spinal muscular atrophy. CGT is being explored for new diseases such as hemophilia and sickle cell disease. In 2024 alone, up to 21 cell therapy launches and as many as 31 gene therapy launches are expected globally so CGT represents a significant, growing opportunity for the world. There is no doubt that Ireland can contribute to innovation in this space with the ultimate goal of bringing the most advanced treatments to Irish patients.
About the author
Dr. Gillian McMahon did her BSc in Analytical Science from DCU, followed by a PhD in Biopharmaceutical Analysis from the Royal College of Surgeons in Ireland. She spent a summer at the Lombardi Cancer Centre at Georgetown University in the US as part of her doctoral research. She has worked in a number of Pharmaceutical companies e.g. Zeneca (UK) and Bristol-Myers Squibb (Ireland) as a Development Chemist and Analyst. She spent many years lecturing in academia e.g. DCU, RSCI and TUD. She successfully started up and funded her own research group – The Bioanalytical Chemistry and Diagnostics (BCD) group – when she was in DCU. The BCD group focused on developing new diagnostic approaches to disease and new analytical methods for biological samples in collaboration with clinicians in the Mater Hospital and St. Vincent’s Hospital. Dr. McMahon is a published author with a textbook and many research papers in peer-reviewed journals to her name. She is currently a Programme Lead and Lecturer with Innopharma Education.
Was this article interesting? See more interesting insights from Innopharma Education team here.



















